q branch spectroscopy
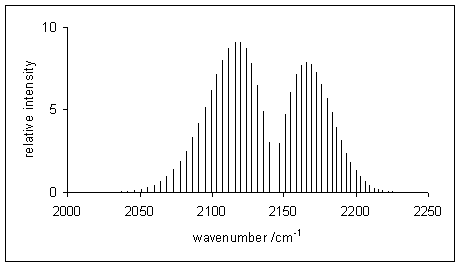
The P-branch is to the left of the gap at 2140 1cm the R. Here were going to as- sume LTE conditions.

Vibrational Spectroscopy The Comparison Between A Classical Harmonic
We therefore return 4 to the Boltzmann distribution of states.

. When such transitions emit or absorb photons the frequency is proportional to the difference in energy. Delta v pm1. Spectral measurement devices are referred to as spectrometers spectrophotometers.
The v0-v1 where delta J0 transition is forbidden for heteronuclear diatomics HCl CO etc due to the selection rules that govern changes in quantum states. Delta J 1 High frequency. Spectroscopy is a branch of science concerned with the spectra of electromagnetic radiation as a function of its wavelength or frequency measured by spectrographic equipment and other techniques in order to obtain information concerning the structure and properties of matter.
Want to thank TFD for its existence. Kyü branch spectroscopy A series of lines in molecular spectra that correspond to changes in the vibrational quantum number with no change in the rotational quantum number. The Q Branch of the v4 Band From 1961 until now only two spectra of the v band of 2CH have been recorded and calibrated.
And I am confused about Q branch which corresponds to delta J 0. Homo-nuclear diatomic molecules do not have an ir spectrum as dmudx 0 as the dipole is zero. Rotationalvibrational spectroscopy is a branch of molecular spectroscopy concerned with infrared and Raman spectra of molecules in the gas phase.
This transition is allowed for perpendicular bands. JOURNAL OF MOLECULAR SPECTROSCOPY 78 170-174 1979 NOTES The High Resolution Spectrum of CH4. McGraw-Hill Dictionary of Scientific Technical Terms 6E Copyright 2003 by The McGraw-Hill Companies Inc.
This chapter is concerned with the relation between the structure of a substance to its rotational and vibration spectra and electron spectra of the bonding electrons. The rule is then Delta J 0. Q branches occur in spherical top molecules like methane.
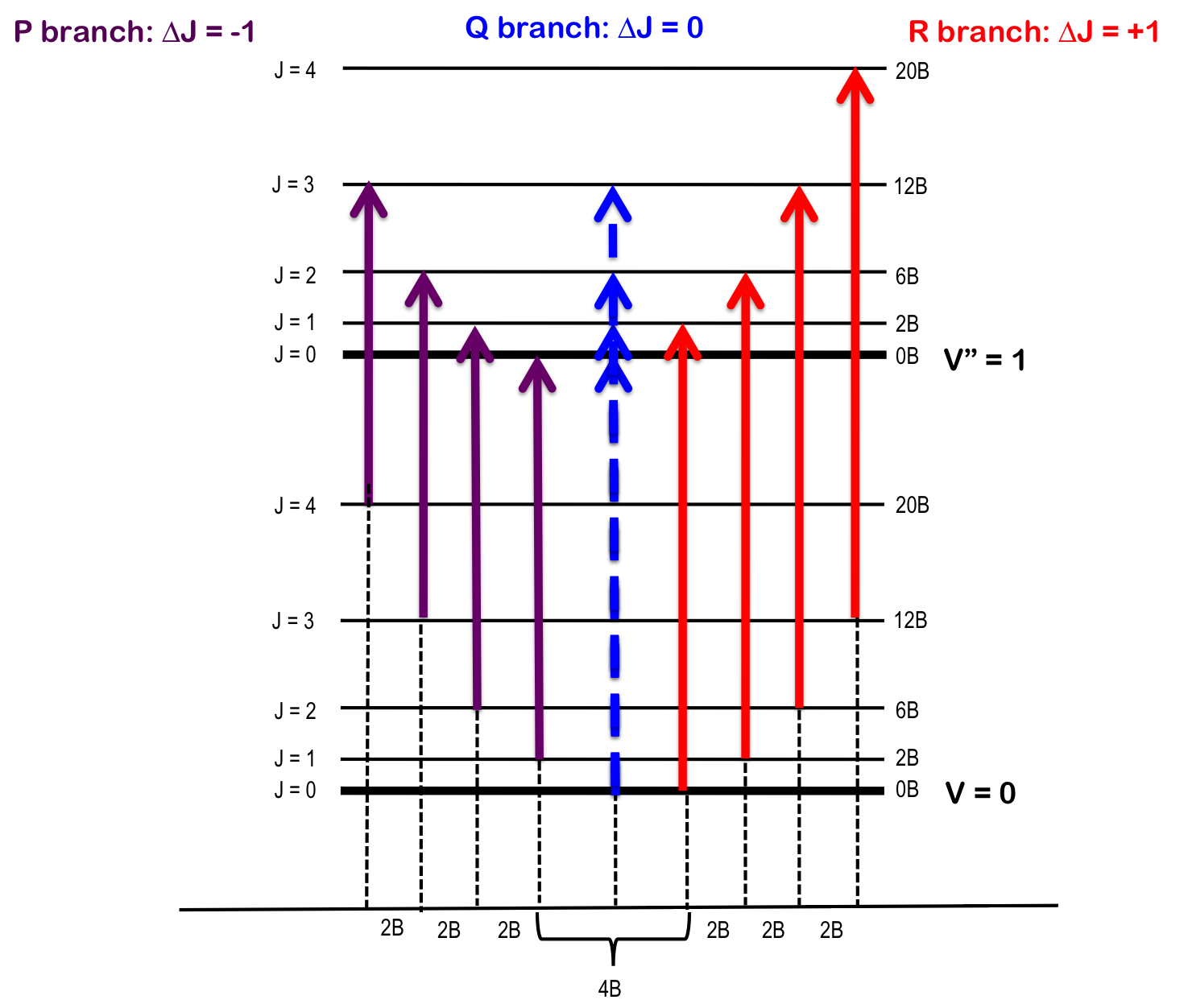
Some of the quantum mechanical foundations of optical spectroscopy were already covered in chapter 3 and the optical methods will be the topic of chapter 7. P branch Q branch E 0 2B 6B 12B J By measuring absorption splittings we can get B. The energy difference is then.
Narahari Rao unpublished with a resolution of about 0200 cm -1 I and the second published by. The Raman Q branch of N 2 has been recorded at room temperature in the pressure range 1506800 bar which corresponds to densities from 135 to 800 amagat. µq to molecular symmetry axis.
I know certain facts such as the following. The first was obtained by K. The Q branch corresponds to vibrational excitation with no change in the rotational quantum number so v v 1 and J J - 1.
Simulated vibration-rotation line spectrum of carbon monoxide. Thus the central vibrational Raman lines Q branch will be accompanied by two wings or branches. Department of Energys Office of Scientific and Technical Information.
3 In the case where B B this becomes. Intensity of Vibrational-Rotational Transitions. The molecular constants for the Q branch 0110 000 of 16O12C18O have been calculated by a least mean-square fit to lines J1 to 16 to be.
From that the bond length. Vo 6623717 00003 cm-1 ΔB 965 002 x 10-4 cm 1. In this case each transition in the Q branch from every rotational level of the lower vibrational state occurs at the same frequency - the energy of the pure vibrational transition.
Transitions involving changes in both vibrational and rotational states can be abbreviated as rovibrational transitions. Begingroup You are correct in that the electronic angular momentum Lambda ne 0 for a Q branch to be observed in a diatomic molecule ie. Where vo has been fixed in absolute magnitude by assuming the - 16O12C16O line 0220 0110 P7 as a reference standard at 662325 cm.
Delta J 0 no band due to selection rule. Delta J -1 Low frequency. 2014 Self broadened N2 Q-branch spectra are measured by high resolution stimulated Raman spectroscopy in the pressure region 025-19 atm.
The molecule is not in a Sigma state. Im struggling in how to exactly explain the origin of PQ and R bands in an IR spectrum with a vibrating - rotating linear diatomic molecule. Do you need to go deeper than that.
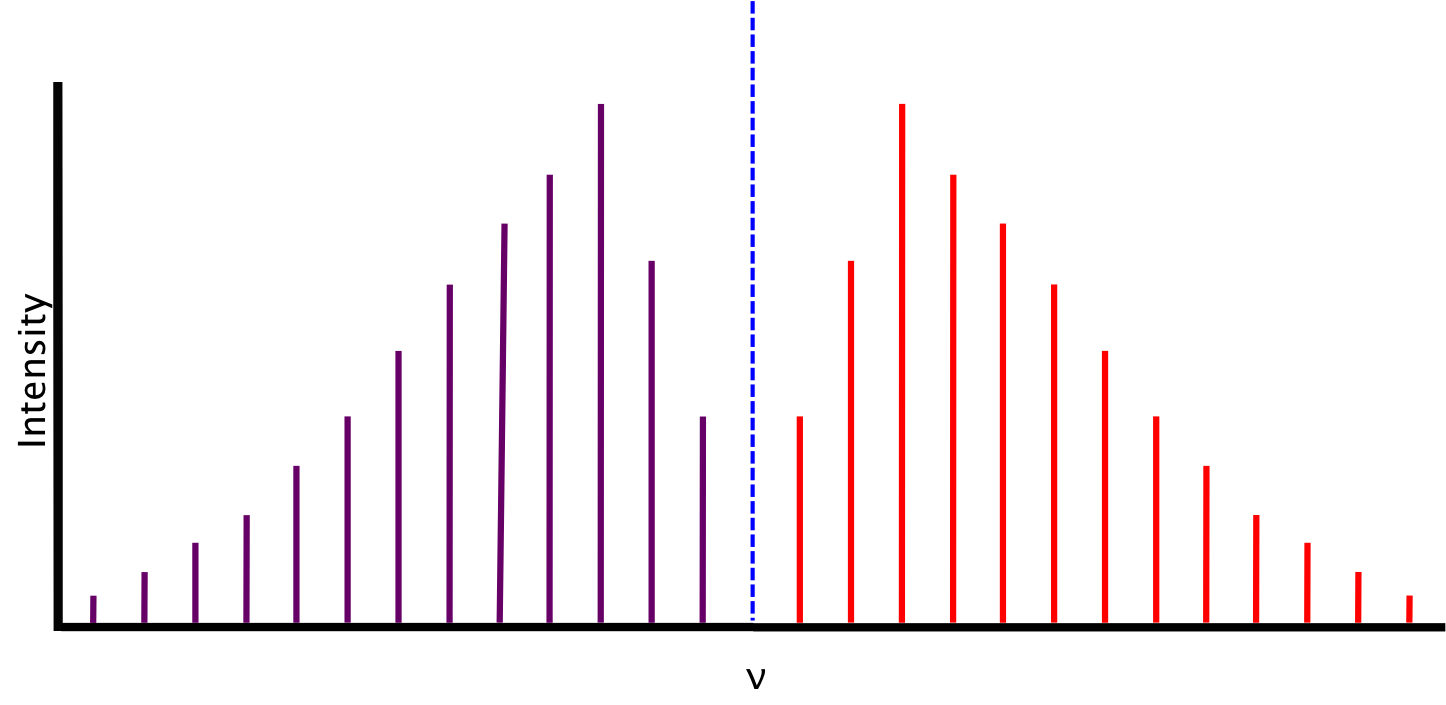
In polyatomics we can also have a Q branch where J0 and all transitions lie at νν0. In this domain the Raman Q branch profile is mainly determined by the well-known collisional narrowing. The energy change of rotation can be either subtracted from or added to the energy change of vibration giving the P- and R- branches of the spectrum respectively.
The transition Delta vpm 1Delta J0 Q-branch is forbidden. The branch on the low frequency side for which J is -2 is known as O branch and that on the high frequency side for which J is2 is called S branch. The experimental data have been obtained by means of a high resolution stimulated Raman.
3 LineStrengths The strengths of spectroscopic lines depends on the probability of the individual transi- tion but also on the population of the states that are involved. In rotational-vibrational spectroscopy P-branch corresponds to low level rotational transitions delta J -1 and R-branch to high rotations delta J 1. The signal is measured employing either a photomultiplier tube low background levelRIPS or a photodetector high background levelIRS and OHD-RIPS.
Which means that no rotational change is there and it is allowed in some cases. The techniques of inverse Raman spectroscopy Raman-induced polarization spectroscopy RIPS and optical heterodyne RIPS OHD-RIPS are compared by probing the Q-branch of the nitrogen molecule.

Rotational Vibrational Spectroscopy Wikiwand
Rotational Vibrational Spectroscopy Wikiwand
Ib Spectroscopy Techniques 黄油上瘾 Butteraddict

Rotational Vibrational Spectroscopy Wikiwand

P And R Branches In An Infrared Spectrum And Q Branch In Dotted Lines Download Scientific Diagram
Fundamental Rovibrational Spectrum Of Co Modeling And Experimental Tools With Prof Magnes

Rotational Vibrational Spectroscopy Wikiwand

Experiment 9 Rotational Vibrational Spectroscopy Introduction
Vibrational Raman Spectra Of Diatomic Molecules Every Science

Rotational Vibrational Spectroscopy Wikiwand
Infrared Spectroscopy On Gases Infrared And Raman Discussion Group Irdg
Walk Through Of Simulating And Fitting A Simple Spectrum
3 9 Spectra Of Gases Chemistry Libretexts

P And R Branches In An Infrared Spectrum And Q Branch In Dotted Lines Download Scientific Diagram

Rotational Spectroscopy Peak Intensities And Spacings Explained Youtube

Rotational Vibrational Spectroscopy Youtube

P And R Branches In An Infrared Spectrum And Q Branch In Dotted Lines Download Scientific Diagram
Walk Through Of Simulating And Fitting A Simple Spectrum





0 Response to "q branch spectroscopy"
Post a Comment